HRS Clinical Research Unit
Description
Under the direction of Prof. Dr Claude Braun and Dr Maike Aurich, this unit, which is supervised and funded by the Hôpitaux Robert Schuman Hospitals, aims to strengthen the management of research projects, facilitate research by doctors and establish and strengthen collaborations with the country’s research institutes. It also ensures that all the legal, administrative and financial aspects of each research project are handled in accordance with current regulations and international quality standards such as Good Clinical Practice. Supervised and funded by the FHRS, clinical research is an integral part of the Hôpitaux Robert Schuman mission, enabling our patients to benefit from innovative diagnostic and therapeutic approaches. Patients who voluntarily agree to take part in a clinical trial may benefit personally, but they also enable many other patients to benefit from treatments that would never have been available if they had not been tested beforehand. Furthermore, patients who agree to take part in a trial are particularly well monitored and supported.
Added value
Thanks to the strong commitment of its doctors, HRS is currently involved in clinical trials, recruiting patients for interventional clinical studies (with drugs or medical devices) or observational studies, which are carried out internally or in collaboration with national industrial (pharmaceutical companies) and academic partners such as the Luxembourg Institute of Health (LIH), the Laboratoire National de la Santé (LNS) or the University of Luxembourg. This highly supervised process follows a precise study protocol and is only carried out under certain conditions.
Status
Active with several projects in progress.


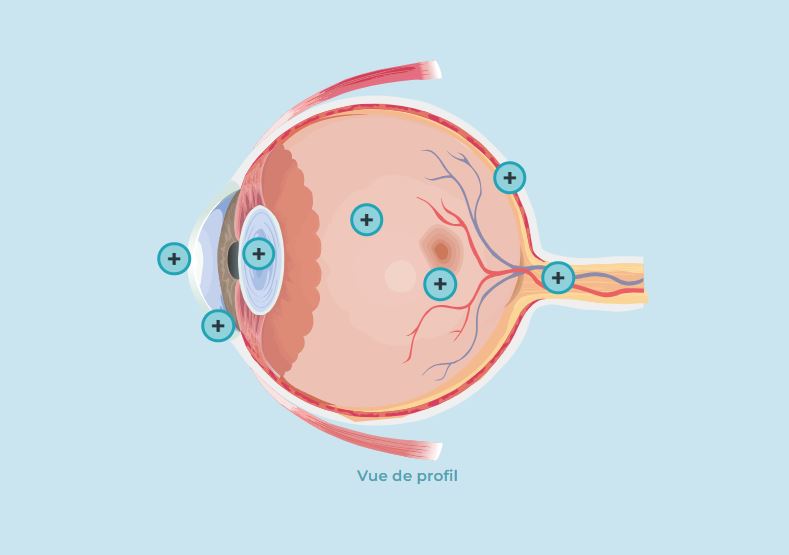
Photo gallery